∞’±≥ß∏È‚Ñ¢ Feeder-Free Pluripotent Stem Cell (PSC) Culture Media
∞’±≥ß∏È‚Ñ¢ Pluripotent Stem Cell Culture Media
Feeder-Free Media for Human ES and iPS Cell Reprogramming, Maintenance, and Differentiation
The TeSR™ family of feeder-free media are produced using rigorously pre-screened materials to ensure the highest levels of batch-to-batch consistency and experimental reproducibility, allowing you to minimize variation in your research. Each medium is based on published formulations1-3 from the laboratory of James Thomson, and allows researchers to maintain high quality human pluripotent stem cell (hPSC) culture systems. These products provide a continuous TeSR™ media-based workflow, from generation of induced pluripotent stem (iPS) cells, to maintenance, differentiation and cryopreservation of embryonic stem (ES) and iPS cells.
Why Use mTeSR™ and the TeSR™ Media Family?
- Feeder-free hPSC culture minimizes variability by limiting the presence of undefined components and immunogenic material.
- m∞’±≥ß∏È‚Ñ¢ is the most widely published medium for hPSC culture with > 1500 peer-reviewed publications.
- The same base formulation in each medium allows for establishment of a continuous TeSR™ media-based workflow.
Choose Your ∞’±≥ß∏È‚Ñ¢ Maintenance Medium
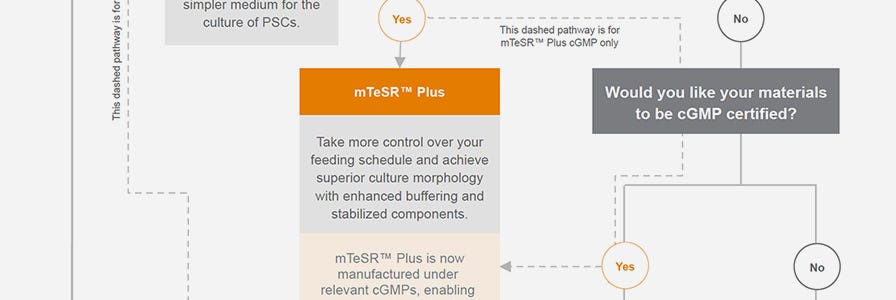
Use the Interactive Product Finder or this infographic to choose the ∞’±≥ß∏È‚Ñ¢ hPSC culture medium that is best suited for your needs.
cGMP hPSC Maintenance Medium
Think forward to the clinic in your hPSC research. Our stabilized feeder-free hPSC maintenance medium, m∞’±≥ß∏È‚Ñ¢ Plus, is now manufactured and tested following relevant cGMPs under a certified quality management system. Find more information about regulatory compliance at ∫£Ω«∆∆Ω‚∞Ê >
How is m∞’±≥ß∏È‚Ñ¢ Plus different from other maintenance media?
m∞’±≥ß∏È‚Ñ¢ Plus was designed based on the formulation of m∞’±≥ß∏È‚Ñ¢1. This version contains stabilized components including FGF2 and unlike other media offers enhanced buffering to reduce medium acidification so that cell quality is preserved during skipped media changes.
Advantages:
- Enhanced buffering and stabilized FGF2 support cell quality while allowing for alternate feeding schedules
- Supports superior culture morphology and cell growth characteristics
- Enables heightened single-cell survival when used with CloneR‚Ñ¢
- Fully compatible with established genome editing and differentiation protocols
- Manufactured under relevant cGMPs, enabling a seamless transition from fundamental research to drug and cell therapy development
Learn more about m∞’±≥ß∏È‚Ñ¢ Plus and try it in your own lab.
Consistent differentiation of ES and iPS cell lines can be challenging. We recommend our ≥ß∞’∑°≤—ªÂæ±¥⁄¥⁄‚Ñ¢ suite of products for optimal and reproducible differentiation.
What Are the Functions of Cytokines in TeSR™ Media and Their Impact on hPSC Culture?
- promotes cell survival and proliferation, while also inhibiting differentiation to specific lineages (e.g. cardiomyocytes). It is present in all of our TeSR™ media (except for TeSR™-E5).
- is an important cytokine for hPSC self-renewal and expansion and can be found in the TeSR™ reprogramming (ReproTeSR™, TeSR™-E7™) and maintenance (, , ) media.
- inhibits reprogramming and is important for maintenance of hPSC pluripotency. ∞’≥“πÛŒ≤ is found in all three TeSR™ maintenance media (, , ).
Brand History
In 2006, Dr. Tenneille Ludwig and colleagues of Dr. James Thomson‚Äôs lab at the University of Wisconsin reported the derivation of a new ES cell line in fully defined, feeder-free culture conditions.1,2 This first defined medium significantly improved human ES cell culture, and was commercially released as m∞’±≥ß∏È‚Ñ¢1, becoming the most widely published feeder-free medium, used in over 1100 peer-reviewed publications. Later, a xeno-free medium based on the same formulation was released as ∞’±≥ß∏È‚Ñ¢2. In 2012, a new, low-protein maintenance medium called ∞’±≥ß∏È‚Ñ¢-E8‚Ñ¢ was released. Based on the E8 formulation3 published by Dr. Guokai Chen of Dr. James Thomson‚Äôs lab, ∞’±≥ß∏È‚Ñ¢-E8‚Ñ¢ contains only the most essential media components required, thereby providing a simpler medium for maintenance of hPSCs.
More recently, m∞’±≥ß∏È‚Ñ¢ Plus (2019) and ∞’±≥ß∏È‚Ñ¢-AOF (Animal Origin-Free; 2021) have been released as part of ∫£Ω«∆∆Ω‚∞Ê Technologies' ∞’±≥ß∏È‚Ñ¢ product line. m∞’±≥ß∏È‚Ñ¢-Plus, a feeder-free maintenance medium, allows for weekend-free schedules and differs from m∞’±≥ß∏È‚Ñ¢1 in its enhanced pH buffering. ∞’±≥ß∏È‚Ñ¢-AOF is guaranteed to be free of human and animal materials to the secondary level of manufacturing, providing users with peace-of-mind around viral safety. m∞’±≥ß∏È‚Ñ¢ Plus, ∞’±≥ß∏È‚Ñ¢-AOF, and m∞’±≥ß∏È‚Ñ¢1 are all manufactured under relevant cGMPs.
In addition to ∞’±≥ß∏È‚Ñ¢ maintenance media, ∫£Ω«∆∆Ω‚∞Ê Technologies has developed ∞’±≥ß∏È‚Ñ¢-based media to support other facets of the pluripotent stem cell research workflow, including media optimized for reprogramming fibroblasts (∞’±≥ß∏È‚Ñ¢-E7‚Ñ¢), reprogramming blood cells and fibroblasts (Repro∞’±≥ß∏È‚Ñ¢), differentiation (∞’±≥ß∏È‚Ñ¢-E6 and ∞’±≥ß∏È‚Ñ¢-E5), and cryopreservation (≥æπÛ∞˘±≥ß∏È‚Ñ¢ and πÛ∞˘±≥ß∏È‚Ñ¢-≥ß).
Scientific Resources
Quality Control for Pluripotent Stem Cells
Get to know the key quality attributes of hPSC cultures, including techniques for maintaining and assessing genomic integrity, pluripotency, and morphology.
Panel: Challenges in Ensuring hPSC Quality
Hear global experts discuss key issues impacting the use of human pluripotent stem cells in this series of webinars provided in partnership with Nature Research.
Explore more helpful resources for your hPSC research in our pluripotent resource centers and cell culture methods library
Key Applications
Toxicity Testing with Human iPS Cells
Kleinstreuer NC, Smith AM, West PR, Conard KR, Fontaine BR, Weir-Hauptman AM, Palmer JA, Knudsen TB, Dix DJ, Donley ELR and Cezar GG (2011), , Toxicology and Applied Pharmacology., November, 2011. Vol. 257(1), pp. 111-121.
Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC and Wu JC (2013), Circulation., April, 2013. Vol. 127(16), pp. 1677-1691.
Liu J, Sun N, Bruce MA, Wu JC and Butte MJ (2012), , PLoS ONE., May, 2012. Vol. 7(5), pp. e37559.
Mehta A, Chung YY, Ng A, Iskandar F, Atan S, Wei H, Dusting G, Sun W, Wong P and Shim W (2011), , Cardiovascular Research., September, 2011. Vol. 91(4), pp. 577-586.
Differentiating to Hematopoietic Cells
Carpenter L, Malladi R, Yang C-T, French A, Pilkington KJ, Forsey RW, Sloane-Stanley J, Silk KM, Davies TJ, Fairchild PJ, Enver T and Watt SM (2011), , Blood., April, 2011. Vol. 117(15), pp. 4008-4011.
Dravid G, Zhu Y, Scholes J, Evseenko D and Crooks GM (2011), , Mol Ther. Vol. 19, pp. 768-81.
Niwa A, Heike T, Umeda K, Oshima K, Kato I, Sakai H, Suemori H, Nakahata T and Saito MK (2011), , PLoS One. Vol. 6, pp. e22261.
Salvagiotto G, Burton S, Daigh CA, Rajesh D, Slukvin II and Seay NJ (2011), , PLoS ONE., March, 2011. Vol. 6(3), pp. e17829.
Differentiating to Definitive Endoderm
Jaramillo M and Banerjee I (2012), , Journal of Visualized Experiments., March, 2012. (61)
Miki T, Ring A and Gerlach J (2011), , Tissue Eng Part C Methods. Vol. 17, pp. 557-68.
Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Lau FH, Musunuru K, Cowan C and Rajagopal J (2012), , Cell Stem Cell., April, 2012. Vol. 10(4), pp. 385-397.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF and Wells JM (2011), , Nature., February, 2011. Vol. 470(7332), pp. 105-109.
Scale-Up and Bioreactor Culture
Oh SKW, Chen AK, Mok Y, Chen X, Lim U-M, Chin A, Choo ABH and Reuveny S (2009), , Stem Cell Research., May, 2009. Vol. 2(3), pp. 219-230.
Olmer R, Haase A, Merkert S, Cui W, Palecek J, Ran C, Kirschning A, Scheper T, Glage S, Miller K, Curnow EC, Hayes ES and Martin U (2010), , Stem Cell Res. Vol. 5, pp. 51-64.
Singh H, Mok P, Balakrishnan T, Rahmat SN and Zweigerdt R (2010), , Stem Cell Res. Vol. 4, pp. 165-79.
Zweigerdt R, Olmer R, Singh H, Haverich A and Martin U (2011), , Nature Protocols. Vol. 6(5), pp. 689-700.
Differentiating to Cardiomyocytes
Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL and Palecek SP (2012), , International Journal of Cell Biology. Vol. 2012, pp. 1-13.
Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ and Palecek SP (2012), , Proceedings of the National Academy of Sciences., July, 2012. Vol. 109(27), pp. 10759-10760.
Mehta A, Chung YY, Ng A, Iskandar F, Atan S, Wei H, Dusting G, Sun W, Wong P and Shim W (2011), , Cardiovascular Research., September, 2011. Vol. 91(4), pp. 577-586.
Zhang H, Zou B, Yu H, Moretti A, Wang X, Yan W, Babcock JJ, Bellin M, McManus OB, Tomaselli G, Nan F, Laugwitz K-L and Li M (2012), , Proceedings of the National Academy of Sciences., July, 2012. Vol. 109(29), pp. 11866-11871.
References
- Ludwig TE et al. (2006) Feeder-independent culture of human embryonic stem cells. Nat Methods 3(8): 637–46.
- Ludwig TE et al. (2006) Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24(2): 185–7.
- Chen G et al. (2011) Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8(5): 424–9.