Protocol for Total RNA Isolation from Cells
- Document # 27175
- Version 1.0.0
- Dec 2019
The following protocol is for total RNA isolation from cells using the Total RNA Purification Kit (Catalog #79040). For complete instructions, refer to the Technical Manual (Document #10000005434).
Directions
A. Preparation of Cell Lysate
Prepare cell lysate from adherent cells or cells in suspension, as indicated below.
From Adherent Cells
- Aspirate cell culture medium. Add ice-cold sterile D-PBS as indicated in Table 1. Aspirate D-PBS.
- Add RNA Lysis Buffer + TG as indicated in Table 1. Gently rock the plate or flask to completely cover the adherent cells with buffer. Pipette the lysate up and down over the cultureware surface 7 ã 10 times.
- Collect the lysate and transfer to a new microcentrifuge tube.
- Add 100% isopropanol as indicated in Table 1. Mix by vortexing for 5 seconds.
- Proceed to RNA isolation.
Table 1. Recommended Volumes of PBS, RNA Lysis Buffer + TG, and 100% Isopropanol for Various Cultureware
From Cells in Suspension
- In a sterile centrifuge tube, centrifuge cell suspension at 300 x g for 5 minutes. Remove and discard supernatant.
- Add ice-cold, sterile D-PBS to wash cells. Centrifuge at 300 x g for 5 minutes. Remove as much supernatant as possible and discard.
- Add RNA Lysis Buffer + TG as indicated in Table 2. Mix well by pipetting up and down 7 - 10 times, or by vortexing. For > 2 x 106 cells, pass the lysate through a 20-gauge needle 4 - 5 times to shear the genomic DNA.
- Add 100% isopropanol as indicated in Table 2. Mix by vortexing for 5 seconds.
- Proceed to RNA isolation.
Table 2. Recommended Volumes of RNA Lysis Buffer + TG and Isopropanol per Cell Input Range
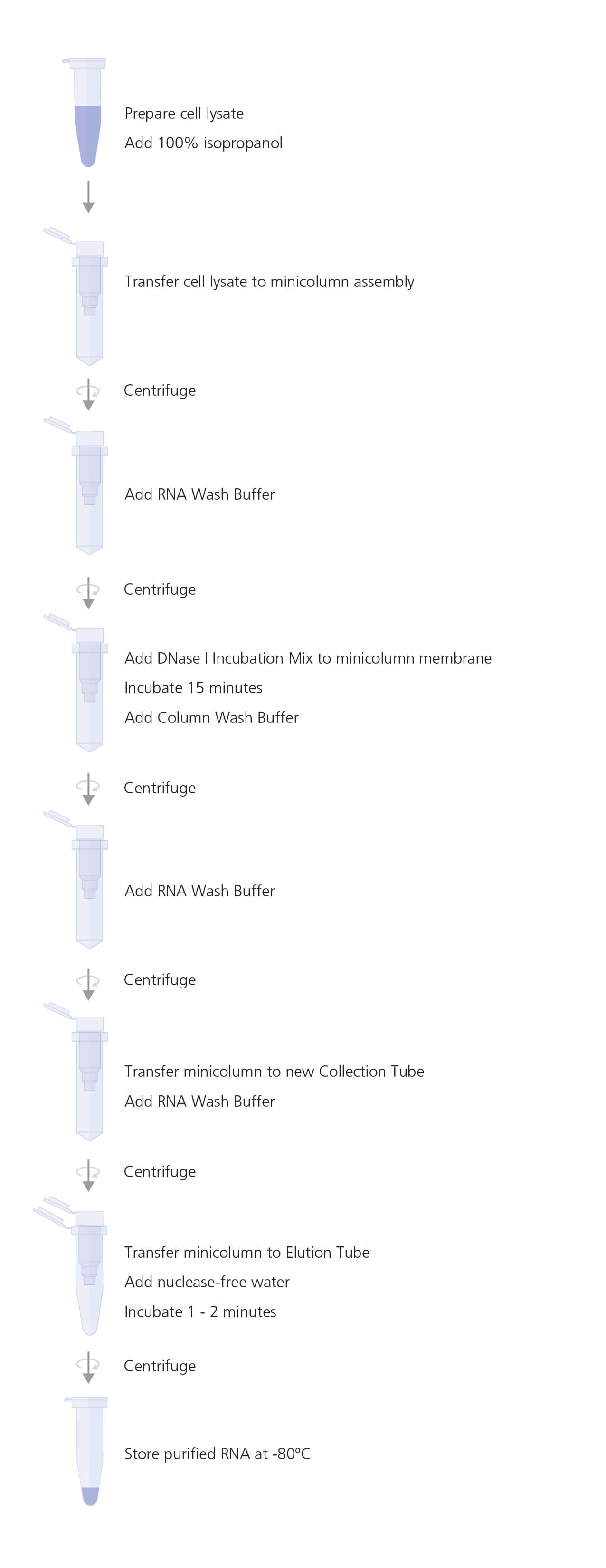
B. RNA Isolation
- Insert minicolumn into Collection Tube.
- Transfer the lysate to the minicolumn assembly.
- Centrifuge at 12,000 - 14,000 x g for 30 seconds. Discard the liquid in the Collection Tube and reinsert minicolumn into Collection Tube.
- Add 500 ö¥L RNA Wash Buffer to the minicolumn. Centrifuge at 12,000 - 14,000 x g for 30 seconds. Discard the liquid in the Collection Tube and reinsert minicolumn into Collection Tube.
- Prepare DNase I Incubation Mix by combining the reagents as indicated in Table 3, per sample, in the order listed.
- Mix by gently pipetting up and down; do not vortex. Store on ice.
- Add 30 ö¥L of fresh DNase I Incubation Mix directly to the minicolumn membrane. Incubate at room temperature (15 - 25ô¯C) for 15 minutes.
- Add 200 ö¥L of Column Wash Buffer (with ethanol added) to the minicolumn. Centrifuge at 12,000 - 14,000 x g for 15 seconds.
- Add 500 ö¥L of RNA Wash Buffer (with ethanol added) to the minicolumn. Centrifuge at 12,000 - 14,000 x g for 30 seconds. Remove the minicolumn and transfer to a new Collection Tube. Discard the Collection Tube containing wash buffer.
- Add 300 ö¥L of RNA Wash Buffer to the minicolumn. Centrifuge at high speed for 2 minutes.
- Transfer minicolumn to an Elution Tube. Add nuclease-free water to the minicolumn membrane as indicated in Table 4. Incubate at room temperature for 1 - 2 minutes. Centrifuge at 12,000 - 14,000 x g for 1 minute.
- Remove and discard minicolumn. Store purified RNA at -80ô¤C.
Table 3. Preparation of DNase I Incubation Mix
Table 4. Recommended Volume of Nuclease-Free Water per Input Cell Range
Genome Editing and Molecular Tools
Find products for genome editing and molecular biology, including the Total RNA Purification Kit.
Molecular Biology Methods Library
Explore more protocols, technical tips, and videos for various molecular biology techniques.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration