How to Co-Culture Human Pluripotent Stem Cell (hPSC)-Derived Forebrain Neurons and Astrocytes
Use this optimized protocol to combine these cell types in 2D after generating each of them separate
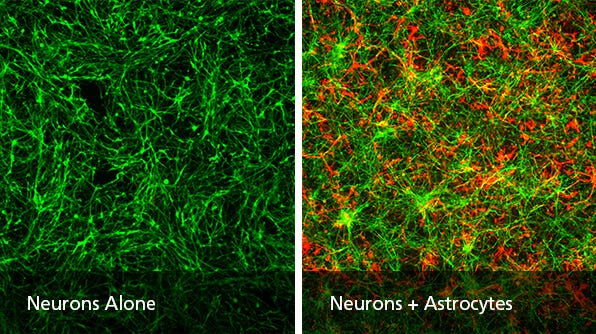
Astrocytes constitute a large portion of the brainãs total cell population and play specialized roles in ion homeostasis, neuronal function, synaptic transmission, blood-brain barrier integrity, and injury response. Astrocytes arise later than neurons during in vivo development as well as during in vitro differentiation protocols and contribute significantly to neural circuit development1. To model relevant neural cell-cell interactions in vitro, it is often advantageous to co-culture astrocytes with neurons. This protocol describes how complex intercellular interactions can be partially recapitulated in two-dimensional culture by separately deriving forebrain-type neurons and astrocytes from human pluripotent stem cells (hPSCs), then combining them into a co-culture.
Materials
- STEMdiffã Forebrain Neuron Differentiation Kit (Catalog #08600)
- STEMdiffã Forebrain Neuron Maturation Kit (Catalog #08605)
- STEMdiffãÂAstrocyte Differentiation Kit (Catalog #100-0013)
- STEMdiffãÂAstrocyte Maturation Kit (Catalog #100-0016)
- STEMdiffã SMADi Neural Induction Kit (Catalog #08581) *ã
- Poly-L-ornithine hydrobromide (PLO) (Sigma P4957) *
- Laminin (Sigma L2020) *
* Required for forebrain-type neuron differentiation as outlined in the STEMdiffã Forebrain Neuron Product Information Sheet.
ã Required for astrocyte differentiation as outlined in the STEMdiffã Astrocyte Product Information Sheet.
Protocol
This procedure has been optimized for use with hPSC maintenance reagents and multiple embryonic stem (ES) and induced pluripotent stem (iPS) cell lines. For upstream protocols and source materials, please see the mTeSRã Plus Technical Manual and the Product Information Sheet for ¤È§úóó§ã¯Ìãs highly quality-controlled Healthy Control Human iPSC Line, Female, SCTi003-A.
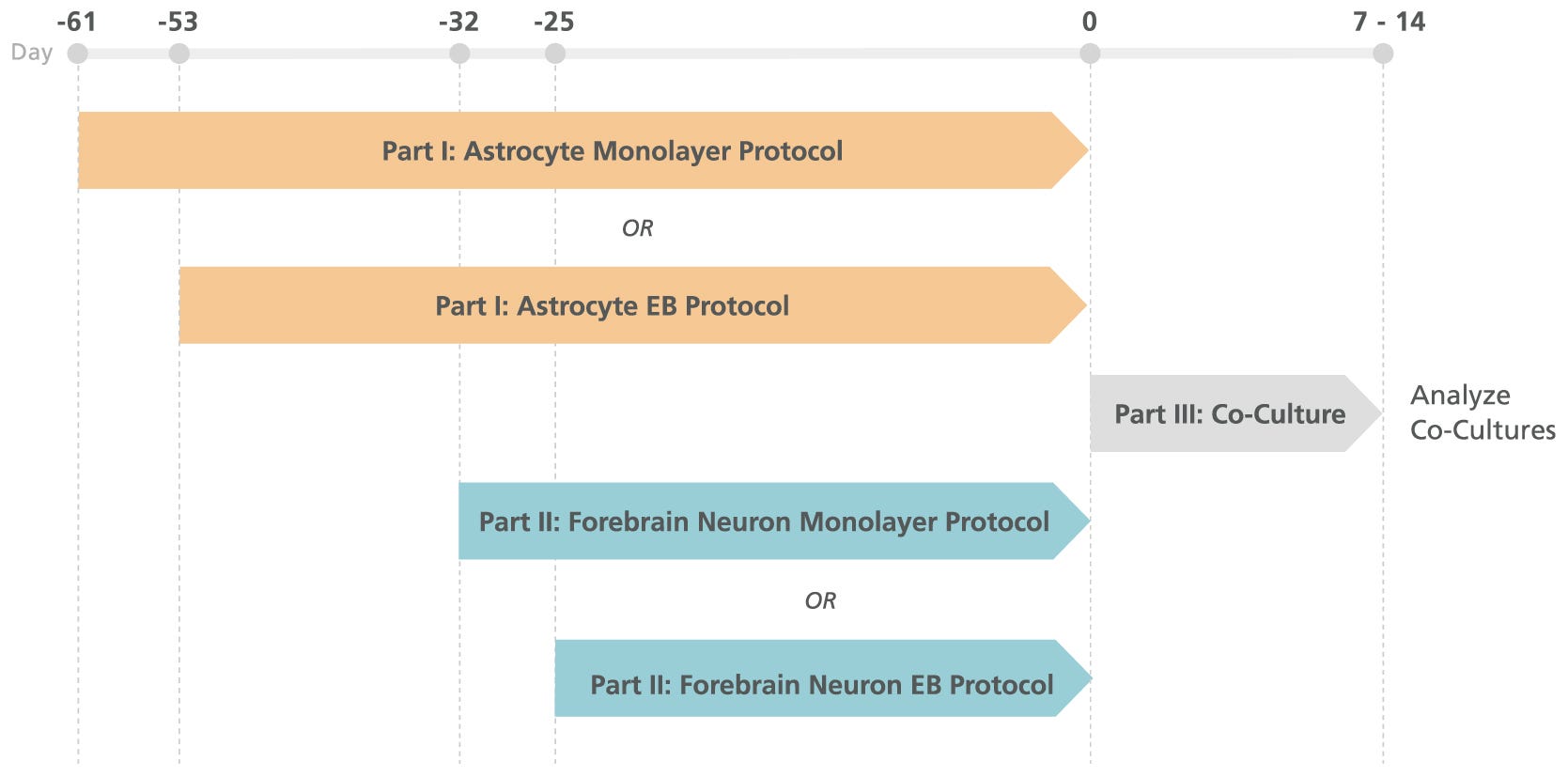
Figure 1. Schematic for Co-Culture of hPSC-Derived Forebrain Neurons with Astrocytes
hPSCs are independently differentiated into forebrain-type neurons and astrocytes that are then combined under optimized co-culture conditions.
Important Note: Prior to combining the cells (at Day 0 in Figure 1), differentiated astrocytes should be cultured in STEMdiffã Astrocyte Maturation Medium for at least 3 weeks, and differentiated forebrain neurons should be cultured in STEMdiffã Forebrain Neuron Maturation Medium for at least 1 week. The timeline depicted in Figure 1 is provided as a general guideline using these maturation periods. If desired, the maturation period for one or both cell types may be extended. As indicated in Figure 1, the timeline will also depend on whether the embryoid body (EB) protocol or monolayer protocol is used for each differentiation. Refer to the relevant Product Information Sheet (PIS) for protocol timelines prior to beginning your experiment. Co-cultures can be maintained for at least 1 - 2 weeks prior to performing analysis.
Part I: Differentiate hPSCs to Astrocytes
- Follow the protocol outlined in the .
- Continue the astrocyte maturation phase until astrocytes have been cultured in STEMdiffã Astrocyte Maturation Medium for at least 3 weeks.
- Verify successful astrocyte differentiation by performing immunocytochemistry on a subset of cultured cells:
Note: With conventional use of the STEMdiffã astrocyte system, the cell population should be > 70% S100õç+, > 60% GFAP+, and < 15% positive for õçIII-tubulin or doublecortin (DCX).
Part II: Differentiate hPSCs to Forebrain Neurons
- Follow the protocol outlined in the .
Note: When seeding neuronal precursors into STEMdiffã Forebrain Neuron Maturation Medium (section C, step 1 of the PIS), the suggested density ranges from 1.5 x 104 - 6 x 104 cells/cm2, depending on the downstream application and intended culture length. The optimal density should be determined by the user. - Continue the forebrain neuron maturation phase until neurons have been cultured in STEMdiffã Forebrain Neuron Maturation Medium for at least 1 week.
- Verify successful forebrain neuron differentiation by performing immunocytochemistry on a subset of cultured cells:
Note: With conventional use of the STEMdiffã forebrain neuron system, the cell population should be > 90% positive for õçIII-tubulin and FOXG1, and < 10% GFAP+.
Part III: Set Up Neuron-Astrocyte Co-Culture
- Dissociate mature astrocytes from Part I according to steps 1 - 5 of section C (Astrocyte Maturation) of the .
Note: Resuspend the cells in an appropriate volume of complete STEMdiffã Astrocyte Maturation Medium, and perform a cell count using Trypan Blue and a hemocytometer. - Dilute the cell suspension in additional complete STEMdiffã Astrocyte Maturation Medium to obtain the required cell concentration and volume.
Note: The concentration and volume of the astrocyte suspension should be optimized by the user and will depend on the desired astrocyte-to-neuron cell-type ratio, the number of culture wells used, and the initial cell density of forebrain neuronal precursors plated in Part II. The recommended astrocyte-to-neuron cell-type ratio ranges from 2:1 to 6:1. - Remove and discard the culture medium from the forebrain neurons generated in Part II.
- Seed the resuspended astrocytes prepared in step 2 onto the forebrain neurons.
- After 1 day, replace the medium with fresh STEMdiffã Forebrain Neuron Maturation Medium.
- Incubate the co-cultures at 37ô¯C and 5% CO2. Perform a full-medium change with STEMdiffã Forebrain Neuron Maturation Medium every 2 - 3 days. Co-cultures can be maintained for at least 1 - 2 weeks prior to analysis.
Reference
- Sloan SA & Barres BA. (2014) Curr Opin Neurobiol 27: 75ã81.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration