How to Remove Granulocytes from Old Blood Samples
How to remove granulocytes by immunodensity cell separation using RosetteSepôÛ Human Granulocyte Depletion Cocktail
Granulocytes change density as blood samples age. This results in granulocyte contamination of mononuclear cells when processing older blood samples (> 24 hours post collection) using a density gradient medium. For effective granulocyte depletion in older human whole peripheral blood samples, immunodensity cell separation can be used to support LymphoprepãÂ- or FicollãÂ-based elimination of granulocytes using density gradient centrifugation.
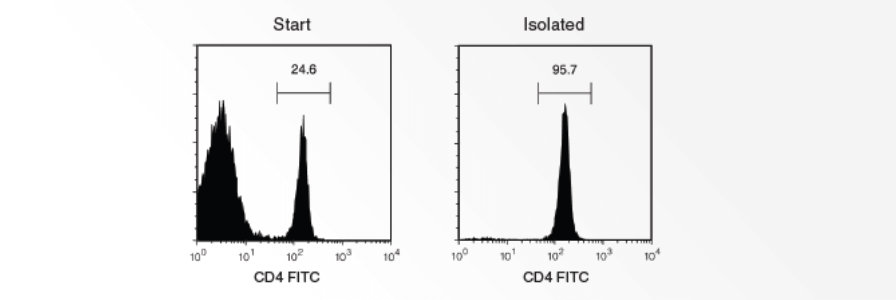
This protocol describes how to remove granulocytes by immunodensity cell separation using RosetteSepôÛ Human Granulocyte Depletion Cocktail. Additionally, a second option is provided in which a SepMateã tube is used to harvest isolated mononuclear cells with a simple pour. Performing immunodensity cell separation before density gradient centrifugation can result in a blood sample containing < 1% granulocytes, compared to 20% granulocytes with density gradient centrifugation alone.
Option 1: Density Gradient Centrifugation Using a Non-SepMateã Tube
Materials
- Whole blood sample collected with anticoagulants
- RosetteSepôÛ Human Granulocyte Depletion Cocktail (Catalog #15624)
- Dulbecco's Phosphate Buffered Saline with 2% Fetal Bovine Serum (PBS + 2% FBS, Catalog #07905)
- Lymphoprepã (Catalog #18060)
- Tube for centrifugation
Protocol
Before You Begin: Ensure that the whole blood sample, PBS + 2% FBS, LymphoprepãÂ, and centrifuge are all at room temperature (15 - 25ô¤C).
- Add RosetteSepôÛ Human Granulocyte Depletion Cocktail at 50 ôçL/mL of whole blood and incubate at room temperature for 20 minutes.
- Dilute whole blood with an equal volume of PBS + 2% FBS and mix gently.
- Layer the diluted sample on top of the LymphoprepãÂ. Be careful to minimize mixing of the density gradient medium and the sample.
- Centrifuge at 1200 x g for 20 minutes at room temperature (with the brake off).
- Remove the enriched cells from the LymphoprepãÂ:plasma interface.
- Wash enriched cells with PBS + 2% FBS.
- Repeat wash step.
Option 2: Density Gradient Centrifugation Using a SepMateã Tube
Materials
- Whole blood sample collected with anticoagulants
- RosetteSepôÛ Human Granulocyte Depletion Cocktail (Catalog #15624)
- Dulbecco's Phosphate Buffered Saline with 2% Fetal Bovine Serum (PBS + 2% FBS, Catalog #07905)
- Lymphoprepã (Catalog #18060)
- SepMateãÂ-15 (Catalog #85415) or SepMateãÂ-50 (Catalog #85450)
Protocol
Before You Begin: Ensure that the whole blood sample, RosetteSepôÛ Human Granulocyte Depletion Cocktail, PBS + 2% FBS, LymphoprepãÂ, and centrifuge are all at room temperature (15 - 25ô¤C).
- Add RosetteSepôÛ Human Granulocyte Depletion Cocktail at 50 ôçL/mL to whole blood and incubate at room temperature for 10 minutes.
- Dilute whole blood with an equal volume of PBS + 2% FBS and mix gently.
- Add Lymphoprepã to the SepMateã tube through the hole in the insert.
- Pipette the diluted sample down the side of the SepMateã tube.
- Centrifuge at 1200 x g for 20 minutes at room temperature (with brake on).
- Pour off the top layer, which contains the enriched mononuclear cells, into a new tube. Do not hold the SepMateã tube in the inverted position for longer than 2 seconds.
- Wash enriched cells with PBS + 2% FBS.
- Repeat wash step.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration